The promise of bioprinting — creating living tissues and organs with a 3D printer — has always felt like something straight out of a science fiction novel, right?
But believe it or not, this revolutionary field is closer to transforming healthcare than you might think! As someone who’s constantly delving into the cutting-edge of tech, I’ve seen firsthand how researchers are making incredible strides, from developing advanced “bioinks” that mimic our body’s own materials to engineering complex structures with unprecedented precision.
Yet, it’s not all smooth sailing; scaling up these creations to a human level and ensuring they function perfectly within our bodies presents some seriously tough technical hurdles.
But don’t worry, the ingenuity being applied to overcome challenges like vascularization — getting blood vessels to grow within printed tissues — and maintaining cell viability is truly inspiring and paving the way for a future where organ shortages could become a relic of the past.
So, if you’re as fascinated as I am about how science is tackling these monumental tasks, let’s dive in and accurately unravel the ingenious solutions scientists are exploring.
What’s up, fellow tech enthusiasts and future-gazing innovators! You know, when I first heard about bioprinting—the idea of 3D printing living tissues and organs—my mind immediately jumped to those classic sci-fi movies.
It just felt so far-fetched, almost like something out of a dream, right? But seriously, the more I dig into this incredible field, the more I’m convinced that it’s not just a dream anymore.
We’re talking about a genuine revolution that’s happening right now, poised to transform healthcare in ways we can barely imagine! As someone who absolutely loves diving headfirst into the latest tech breakthroughs, I’ve been blown away by the progress researchers are making.
From cooking up advanced “bioinks” that basically trick our bodies into thinking they’re the real deal, to crafting super intricate structures with mind-blowing precision, it’s all happening.
But let’s be real, it’s not all sunshine and rainbows. There are some hefty technical mountains to climb, like getting these printed tissues to scale up to human size and ensuring they play nice with our complex biological systems.
And don’t even get me started on making sure those printed tissues get a proper blood supply—we call that vascularization, and it’s a huge deal! But what truly inspires me is the sheer ingenuity being thrown at these problems.
Scientists are working tirelessly to figure out how to keep cells alive and thriving within these printed structures, and how to get blood vessels to grow exactly where they’re needed.
It’s paving the way for a future where maybe, just maybe, organ shortages become a relic of the past. If you’re as fascinated as I am about how science is tackling these monumental tasks, grab a cup of coffee, settle in, and let’s unravel the ingenious solutions scientists are exploring.
The Quest for Smarter Building Blocks: Bioinks That Mimic Life
When you think about 3D printing, you probably picture plastic or metal, right? Well, in bioprinting, our “ink” is a whole different beast. It’s called bioink, and it’s arguably one of the most critical components in this entire endeavor.
Think about it: you’re trying to build something that needs to integrate with a living body, so the material you use needs to be incredibly special. Researchers are constantly pushing the boundaries here, developing bioinks that aren’t just biocompatible, meaning our bodies won’t reject them, but also “bioactive,” which means they can actually interact with cells and encourage them to grow and behave as they would in natural tissue.
We’re talking about hydrogels made from natural polymers like collagen, alginate, and gelatin, which are fantastic because they’re biodegradable and support cell growth beautifully.
But then there’s the challenge: these natural materials can sometimes lack the mechanical strength needed for larger, more complex structures. That’s where the real innovation kicks in!
Scientists are now creating hybrid bioinks, blending natural components with synthetic polymers to get the best of both worlds—strong enough to hold shape, yet still friendly for cells.
It’s like finding that perfect balance where the bioink acts as both a cozy home for cells and a sturdy skeleton for the new tissue. I’ve read about some truly mind-boggling advancements, like using marine-derived biomaterials such as chitosan, which offer cost-effective and sustainable options with excellent mechanical properties and biocompatibility.
They are also developing shear-thinning hydrogels that improve printability, allowing for higher resolution and speed during the printing process. Honestly, watching this field evolve feels like we’re literally designing new life, one tiny, intelligent building block at a time.
It’s incredible to see how these smart materials are moving us closer to creating tissues that are not just printed, but truly *living*.
Crafting the Perfect Environment for Cells
Beyond just the material itself, scientists are meticulously engineering the bioink’s properties to ensure cell survival and functionality. It’s not enough for cells to just *exist* in the bioink; they need to thrive, proliferate, and differentiate into the right cell types.
This means controlling factors like nutrient permeability, water solubility, and even electrical conductivity to provide biochemical and biophysical stimulation, just like in natural tissues.
They’re looking at things like nanoengineered granular hydrogel bioinks to boost mechanical strength while maintaining microporosity, essential for cell attachment and tissue regeneration.
I’ve seen some fascinating work where researchers are using advanced digital controls and innovative nozzle arrays to precisely place multiple spheroids (those spherical cell clusters) simultaneously, ten times faster than older methods, while keeping over 90% cell viability.
Imagine the impact of that speed on creating larger, more complex tissues! This focus on the microenvironment is key, ensuring the cells are happy and productive both during and after the printing process, paving the way for successful tissue formation and integration.
It’s all about giving those little cells the best possible start in their new, bioprinted home.
Next-Gen Bioink Formulations
The horizon for bioinks is brimming with even more exciting possibilities. We’re moving towards formulations that can actively respond to stimuli, like changes in pH or temperature, or even light.
This could mean tissues that can adapt and remodel over time, much like our natural body tissues do. Think about it: a printed tissue that can *heal itself* or grow stronger in response to stress.
We’re talking about dynamic bioinks and even 4D bioprinting, where printed structures can change shape and function over time. Researchers are even exploring decellularized tissue matrices, which are essentially natural tissue scaffolds stripped of their original cells, providing an incredibly biomimetic environment for new cells to colonize.
These advancements are absolutely critical for pushing bioprinting beyond simple tissue patches to truly complex organs that can mimic the intricate functions of their natural counterparts.
It’s a game-changer, allowing for unparalleled control over tissue development and long-term functionality within the body.
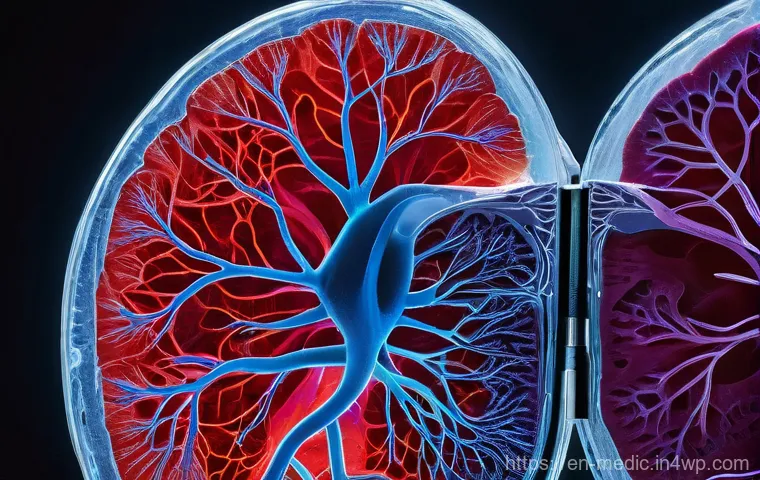
Cracking the Vascularization Code: Bringing Tissues to Life with Blood Flow
Alright, let’s talk about arguably the biggest hurdle in bioprinting functional organs: vascularization. You can print a beautiful, perfectly structured piece of tissue, but if it doesn’t have a reliable blood supply, it’s essentially a fancy paperweight.
Our natural organs are incredibly complex, riddled with an intricate network of blood vessels that deliver oxygen and nutrients while whisking away waste.
Without this, cells simply can’t survive for long, especially in thicker tissues. It’s like building a city without roads or a water supply – it just won’t function.
For me, this was always the part that felt most “science fiction,” but the breakthroughs here are genuinely breathtaking. Scientists are coming up with ingenious strategies to either print blood vessel networks directly or coax printed tissues into developing their own.
One promising approach involves using sacrificial bioinks, which are printed to create channels, and then dissolved away, leaving behind a perfusable network of pathways for blood to flow.
Another exciting area is the integration of microfluidic systems within bioprinted tissues. These tiny channels can help deliver nutrients and oxygen, effectively acting as a preliminary circulatory system until the tissue can develop its own.
I’ve seen demonstrations where these microfluidic systems not only maintain cell viability but also promote the self-assembly of microvascular networks, which is exactly what we need for successful, long-term tissue integration.
This isn’t just about survival; it’s about enabling the printed tissue to truly become a living, breathing part of the body.
Directly Engineering Blood Vessel Networks
One of the most ambitious and impactful approaches involves directly printing vascular structures. Imagine nozzles so fine they can lay down patterns for capillaries, or printing endothelial cells (the cells that line blood vessels) to spontaneously form tubular structures.
Some researchers are using co-axial printing techniques to create multi-layered blood vessels with different diameters, mimicking the varying sizes found in the body.
There’s also the concept of pre-vascularized constructs, where scientists create small, vascularized tissue modules that can then be assembled into larger organs.
This is a bit like building with LEGOs, but each LEGO block already has its own mini road system. These modules can then be integrated, and their vascular networks connected, to form a more complex and larger organ with a functional blood supply.
This approach directly tackles the “too thick” problem that plagued early bioprinting efforts, allowing for the creation of larger constructs that can receive the vital blood flow they need.
Inducing Natural Vascular Growth
Another fascinating strategy is to encourage the printed tissue to grow its *own* blood vessels after implantation. This often involves incorporating angiogenic factors—special proteins that stimulate blood vessel growth—into the bioink or scaffold.
By strategically releasing these factors, scientists can essentially send a signal to the surrounding host tissue, telling it to extend its blood vessels into the new bioprinted construct.
It’s a brilliant way to leverage the body’s natural healing and growth mechanisms. Think of it as planting a seed and giving it the right nutrients to flourish.
I’ve read about studies where such techniques have led to the development of functional vascular networks within transplanted tissues, leading to improved nutrient support and enhanced organ function.
This combined approach of engineering vascularization and inducing it naturally is what’s truly accelerating our progress toward creating functional, clinically viable organs.
It’s a complex dance between technology and biology, and honestly, the sheer cleverness of it all is inspiring.
Precision and Resolution: The Art of Micro-Scale Printing
When we talk about bioprinting, we’re not just talking about big shapes; we’re talking about incredible, almost invisible, detail. The human body is a masterpiece of micro-architecture, and to truly replicate tissues and organs, we need printers that can work at a resolution that’s frankly mind-blowing.
Imagine printing something with features smaller than a human hair, and doing it consistently, layer after tiny layer. That’s the challenge of precision and resolution in bioprinting.
Initially, printers struggled with this, leading to constructs that were structurally okay but lacked the fine details needed for complex biological functions.
But let me tell you, the advancements here have been nothing short of spectacular! We’re seeing printers now capable of sub-micron resolution, which means they can place cells and bioink with astonishing accuracy, creating intricate 3D microenvironments that truly mimic native tissue.
I’ve personally been following the developments in technologies like tomographic volumetric bioprinting and freeform reversible embedding of suspended hydrogels (FRESH), which significantly reduce printing time while enhancing precision.
It’s a bit like comparing a crayon drawing to a hyper-realistic oil painting – the level of detail is just incomparable, and it opens up a whole new world of possibilities for what we can create.
Refining the Printing Process for Detail
Achieving this level of precision isn’t just about smaller nozzles; it involves a whole suite of technological innovations. Researchers are developing new extrusion techniques, like those using miniaturized progressive cavity pumps (PCPs), which offer significantly higher accuracy and precision compared to traditional pneumatic systems.
This means more consistent flow rates of bioink, fewer defects in the printed construct, and ultimately, a more reliable and reproducible product. The beauty of these advancements is that they allow for greater freedom in bioink design, meaning we can use more complex bioink compositions without worrying about clogging or uneven material flow.
We’re also seeing the integration of sophisticated digital control systems that can precisely coordinate multi-axis movements, ensuring that each layer of bioink is placed exactly where it needs to be.
This meticulous control is absolutely essential when you’re trying to create delicate structures like neural interfaces or vascular networks, where even a slight misalignment can compromise functionality.
Multi-Material Magic for Complex Structures
One of the most exciting aspects of enhanced precision is the ability to perform multi-material printing. Our natural tissues aren’t made of just one type of cell or material; they’re incredibly heterogeneous, with different cell types and extracellular matrix components arranged in precise spatial relationships.
With high-resolution multi-material printers, we can now deposit different bioinks, each containing specific cell types or biomolecules, in exact locations.
This means we can create constructs that truly mimic the complexity of native tissues, like printing a vascular network alongside parenchymal cells to create a functional liver lobule.
It’s also boosting printing speed, which is a huge bonus when you’re trying to build something as complex as an entire organ. The ability to spatially control these diverse components with such accuracy is a massive leap forward, allowing us to build biomimetic structures that are not just aesthetically similar but functionally identical to the real thing.
It’s opening doors to creating tissues and organs that don’t just look the part, but truly *act* the part, too.
Nurturing Printed Tissues: Ensuring Cell Viability and Long-Term Function
Let’s face it, printing something is one thing, but making sure it actually *lives* and *functions* like natural tissue in the long run? That’s the real challenge, and it’s something I often think about.
It’s not enough for the cells to survive the printing process; they need to stay viable, differentiate correctly, and integrate seamlessly into the body to perform their intended function.
This is where the magic of post-printing maturation comes in. Imagine taking a freshly printed piece of tissue, and then giving it the perfect “training” environment to grow stronger and more functional.
That’s essentially what researchers are trying to achieve, and it’s a crucial step before any of these incredible bioprinted constructs can ever make it into a patient.
We’re talking about developing advanced bioreactor technologies that can provide the ideal mechanical, chemical, and biological cues for tissue maturation.
This includes ensuring proper nutrient delivery, waste removal, and even applying physical stimulation to encourage cells to develop proper tissue architecture and mechanical properties.
I’ve seen some incredible work on bioreactors that are designed to mimic the dynamic environment within the human body, providing essential serum-free media and controlled conditions to help the cells thrive.
It’s all about making sure these tiny biological factories are working at peak performance.
Keeping Cells Happy During and After Printing
The journey from a cell suspension to a functional tissue is fraught with peril for our precious cells. The printing process itself can be stressful, with shear forces and varying temperatures potentially damaging delicate cells.
That’s why researchers are constantly optimizing printing parameters, developing gentler techniques, and creating bioinks that offer a protective environment.
But even after printing, the cells need a continuous supply of oxygen and nutrients, and efficient removal of waste products. This is where the vascularization strategies we discussed earlier become absolutely critical.
Without a robust blood supply, cells in the interior of a thicker tissue will simply starve and die. Beyond that, the bioink itself plays a huge role.
It needs to be permeable enough to allow for gas and nutrient exchange, and it needs to degrade at a rate that allows cells to gradually remodel their own extracellular matrix, essentially building their own natural support structure.
The ongoing research into bioinks that can release growth factors over extended periods is a significant step forward, providing long-term support for cell differentiation and tissue development.
It’s a testament to how every piece of the bioprinting puzzle interlocks, from the initial ink formulation to the final maturation.
Post-Printing Maturation and Integration
Once a tissue is printed, the work isn’t over. In fact, in many ways, it’s just beginning. The printed construct often needs a period of maturation in a controlled environment, like a specialized bioreactor, to develop full functionality.
During this phase, cells can further differentiate, form proper intercellular connections, and remodel their surrounding matrix, making the tissue stronger and more robust.
This is also where the challenge of seamless integration with the host body comes into play. If the bioprinted tissue is derived from the patient’s own cells—autologous cells—the risk of immune rejection is significantly reduced, which is a massive advantage.
I’m personally excited about the potential of using induced pluripotent stem cells (iPSCs), which can be reprogrammed from a patient’s own adult cells and then differentiated into various cell types needed for a specific organ.
This patient-specific approach not only minimizes rejection risks but also allows for truly personalized medicine, tailoring treatments to individual needs.
The ultimate goal is for these bioprinted tissues to not just survive but to actively participate in the body’s functions, leading to long-lasting and effective therapeutic outcomes.
Scaling Up for Human Needs: From Lab Bench to Clinical Reality

You know, it’s one thing to print a tiny piece of tissue in a lab, barely visible to the naked eye. It’s an entirely different beast to produce a human-sized organ, complete with all its intricate functions, in a way that can actually help real patients.
This “scaling up” is a colossal challenge, one that’s constantly on my mind when I think about bioprinting’s future. It’s not just about making things bigger; it’s about maintaining cell viability during longer printing times, ensuring structural integrity, and overcoming logistical nightmares like storage and transportation.
The sheer number of cells required for a human organ—sometimes billions!—is staggering, and expanding those cells in a lab while maintaining their quality is a massive undertaking.
Honestly, the idea of moving from a petri dish experiment to a full-scale transplant operation feels like jumping from a puddle to an ocean, but the ingenuity being applied is genuinely inspiring.
We’re talking about developing high-throughput bioprinting systems that can churn out tissues at speeds much faster than existing methods while still maintaining high cell viability.
It’s an uphill battle, but one that researchers are tackling with incredible dedication.
Bridging the Gap to Mass Production
For bioprinting to truly revolutionize healthcare, it needs to move beyond bespoke laboratory creations and into a realm of scalable production. This means addressing challenges like the slowness of current printing processes, which are simply not feasible for human-scale constructs.
Innovations in bioprinter design are focusing on increasing speed without sacrificing resolution or cell viability. For instance, new technologies like the “High-throughput Integrated Tissue Fabrication System for Bioprinting” (HITS-Bio) are making it possible to print cartilage tissue ten times faster with over 90% cell viability.
Furthermore, we need standardized protocols and automated systems to ensure reproducibility and consistency across different batches of printed tissues.
Think about the pharmaceutical industry, where every pill is identical. We need a similar level of rigor for bioprinted organs. This also extends to the bioinks themselves, as issues like batch-to-batch variation and the need for GMP-grade (Good Manufacturing Practice) materials are critical for clinical translation.
Without these robust manufacturing standards, large-scale clinical application remains a distant dream.
Navigating the Regulatory Labyrinth
Beyond the technical hurdles, there’s the complex world of regulatory approval. As you can imagine, bringing a living, bioprinted product to market is a far cry from launching a new app.
Regulatory bodies like the FDA in the United States and the EMA in Europe are grappling with how to classify and evaluate these novel products. Are they medical devices?
Biologics? Drugs? Or a combination of all three?
Each classification comes with its own stringent requirements for safety, efficacy, and quality. This creates a “regulatory limbo” that developers need to navigate carefully.
I’ve been following discussions about the need for clearer guidelines and flexible frameworks that can adapt to the rapid advancements in this field. It’s a massive undertaking, requiring collaboration between scientists, engineers, and policymakers to establish robust quality frameworks, ensure patient safety, and address ethical considerations.
Until these pathways are well-defined and streamlined, the journey from laboratory breakthrough to widespread clinical availability will remain a challenging one, but it’s a vital step to truly unlock bioprinting’s full potential.
Ethical Lenses and Societal Impact: A Deeper Look at Bioprinting’s Promise
Okay, so we’ve talked a lot about the incredible science and engineering behind bioprinting, and it’s truly awe-inspiring. But there’s another, equally important, side to this coin: the ethical and societal implications.
As someone who’s always thinking about how technology shapes our world, I find this area absolutely fascinating, and a little daunting, too. When we talk about creating living tissues and organs in a lab, we’re stepping into territory that raises profound questions about what it means to be human, the very nature of life, and how we ensure equitable access to these life-changing technologies.
It’s not just about what *can* be done, but what *should* be done, and for whom. The potential for bioprinting to solve organ shortages is immense, offering hope to millions on transplant waiting lists.
But what about the ethical sourcing of cells, especially when embryonic stem cells are involved (though iPSCs offer a promising alternative)? These aren’t simple questions with easy answers, and they’re discussions that we, as a society, need to have openly and thoughtfully as this technology continues to advance at breakneck speed.
Navigating the Moral Maze of Cellular Sourcing
One of the first ethical considerations that jumps out at me is where the biological materials—especially the cells—come from. While induced pluripotent stem cells (iPSCs), derived from a patient’s own adult cells, offer a way to bypass some controversies, the historical use of embryonic stem cells (ESCs) in research has sparked significant debate.
The destruction of human embryos for ESCs raises fundamental questions about the beginning of human life and moral status. Even with iPSCs, there are discussions around their safety, given they are reprogrammed cells that haven’t been widely tested in human transplantation.
Then there’s the issue of donor consent and confidentiality if allogeneic (non-patient specific) cells are used. It’s a delicate balance, trying to push the boundaries of medical science while respecting deeply held beliefs and ensuring the highest ethical standards are maintained at every step.
I personally believe transparency and rigorous ethical oversight are absolutely paramount here to build public trust and ensure responsible innovation.
Equity, Access, and the Future of Healthcare
Perhaps the biggest societal question for me revolves around equal access. If bioprinted organs become a reality, who will benefit? Will this be a luxury available only to the wealthy, or will it be a widely accessible solution that truly addresses global health disparities?
We’ve seen how often cutting-edge medical technologies can become financially exclusive, and it’s a critical concern that needs to be proactively addressed *now*, before bioprinting becomes commonplace.
Considerations also extend to the concept of human identity. As bioprinted organs become more sophisticated, mirroring natural ones, it might blur the lines between what’s “natural” and “artificial,” leading to philosophical debates about the “digitization” of the human body.
And what about the potential for human enhancement, not just therapy? These are complex, future-facing discussions, but they underscore the need for a robust ethical framework that considers every facet of bioprinting’s impact.
Ultimately, ensuring that bioprinting serves humanity’s best interests, ethically and equitably, is as important as the scientific breakthroughs themselves.
| Challenge Area | Key Technical Hurdles | Innovative Solutions & Advancements | Impact on Bioprinting |
|---|---|---|---|
| Bioink Development | Biocompatibility, mechanical strength, printability, cell viability. | Hybrid bioinks, shear-thinning hydrogels, smart materials (stimuli-responsive), decellularized ECM, marine-derived polymers. | Enables creation of complex, functional tissues with improved cell integration and long-term viability. |
| Vascularization | Nutrient/oxygen delivery, waste removal, recreating intricate blood vessel networks in thick tissues. | Sacrificial bioinks, microfluidic systems, direct printing of vascular scaffolds, angiogenic growth factor incorporation, pre-vascularized tissue modules. | Crucial for survival and function of larger bioprinted tissues and organs; reduces cell death. |
| Precision & Resolution | Accurate cell placement, recreating micro-architecture, multi-material deposition, printing speed. | PCP extrusion systems, tomographic volumetric bioprinting, multi-axis control, high-throughput systems (HITS-Bio), sub-micron resolution printers. | Allows for creation of highly detailed, biomimetic structures critical for complex organ functionality. |
| Cell Viability & Functionality | Maintaining cell health during/after printing, differentiation, long-term integration, post-printing maturation. | Optimized printing parameters, protective bioinks, advanced bioreactor technologies, iPSCs for patient-specific tissues. | Ensures bioprinted constructs remain living, functional, and integrate effectively with the host body. |
| Scalability & Clinical Translation | Mass production, lengthy printing times, logistics (storage/transport), regulatory approval, cost. | High-throughput bioprinting, standardized protocols, GMP-grade bioinks, collaborative regulatory dialogues, AI optimization. | Moves bioprinting from lab research to widespread clinical application for addressing organ shortages. |
Future Horizons: What’s Next for Bioprinting?
So, we’ve taken a deep dive into the incredible progress and persistent challenges of bioprinting, and I don’t know about you, but I’m absolutely buzzing with excitement for what’s next!
It truly feels like we’re standing at the precipice of a medical revolution, where the promise of creating patient-specific tissues and organs on demand is slowly but surely becoming a tangible reality.
The impact this could have on healthcare, from eliminating organ waitlists to revolutionizing drug discovery, is almost too vast to comprehend. Imagine a future where a damaged heart can be replaced with one printed from your own cells, perfectly matched and free from rejection risks.
It’s not just about organs, either. Bioprinting is already being explored for everything from advanced wound healing and skin grafts to drug testing platforms that can provide more accurate results than animal models, reducing ethical concerns there too.
The journey is far from over, but the ingenuity and dedication of researchers worldwide are truly paving the way for a future that once belonged only to the pages of science fiction.
Personalized Medicine Takes Center Stage
One of the most thrilling prospects, in my opinion, is the ultimate realization of personalized medicine through bioprinting. The ability to use a patient’s own cells (like iPSCs) to create custom tissues and organs means a virtually eliminated risk of immune rejection, which is a monumental problem in traditional transplantation.
This isn’t just about avoiding rejection; it’s about crafting a solution that is perfectly tailored to an individual’s unique physiology and needs. Think about it: a specific patch of bone to repair an injury, or a section of cartilage perfectly designed to integrate with a damaged joint.
This level of customization opens up a world where treatments are not one-size-fits-all but are as unique as the patients themselves. I’ve read about personalized artificial tracheas and auricular cartilage being fabricated, showing promising functional and aesthetic results.
The potential for enhancing surgical outcomes and developing bespoke therapies is truly limitless.
Beyond Replacements: Bioprinting’s Broader Impact
While organ replacement grabs the headlines, bioprinting’s reach extends far beyond that. We’re already seeing its significant impact in pharmaceutical development, creating “organ-on-a-chip” models that are realistic 3D replicas of human organs.
These models allow researchers to test new drugs and therapies with unprecedented accuracy, predicting efficacy and toxicity much more reliably than traditional methods.
This not only accelerates drug discovery but also reduces the reliance on animal testing, which is a huge win for both science and ethics. Furthermore, bioprinting is making strides in creating advanced disease models, offering a clearer window into how diseases progress and how we might combat them.
The convergence of bioprinting with other cutting-edge technologies, like artificial intelligence and machine learning, is also incredibly exciting. AI can help design complex tissue structures and predict cell behavior, further accelerating development.
It’s a holistic approach, combining biology, engineering, and digital intelligence to forge a healthier future for everyone. It’s a journey, for sure, but one that promises to reshape the very landscape of medicine as we know it.
글을마치며
Whew! What a journey we’ve taken through the incredible world of bioprinting! Honestly, diving into this topic always leaves me feeling a mix of awe and pure excitement. It’s truly mind-boggling to think that we’re on the cusp of a future where organ shortages could be a thing of the past, and personalized medicine takes on a whole new, tangible meaning. The breakthroughs happening right now, from those super clever bioinks to the intricate dance of vascularization, are not just science fiction dreams anymore—they’re becoming our reality, one tiny, perfectly placed cell at a time. It’s a powerful reminder that human ingenuity, when focused on solving humanity’s biggest challenges, truly knows no bounds!
알아두면 쓸모 있는 정보
1. Keep an Eye on the “Bio-Revolution” Beyond Organs: While organ printing grabs the headlines, remember that bioprinting is quietly revolutionizing other areas too! Think about personalized drug testing – creating tiny, functional human organ models on a chip means drug companies can test medications with far greater accuracy and speed, potentially reducing side effects and getting life-saving treatments to us faster. It also significantly cuts down on animal testing, which is a huge win for everyone. I’ve been following some really exciting developments in bioprinted skin for burn victims, and even corneal tissues to help restore sight. The scope is just massive!
2. Your Cells, Your Future: The Power of iPSCs: Ever heard of induced pluripotent stem cells (iPSCs)? They’re game-changers! Scientists can now take ordinary adult cells from *you*—say, a skin cell—and reprogram them to act like embryonic stem cells. This means they can then differentiate into almost any cell type your body needs, from heart cells to liver cells. The coolest part? If we can use your own iPSCs to print new tissues or organs, the risk of your body rejecting them essentially vanishes! It’s the ultimate form of personalized medicine, making your own body the blueprint for your repair kit. This innovation addresses so many ethical concerns that arose from using embryonic stem cells and truly brings us closer to a patient-specific healthcare paradigm.
3. The “4D Bioprinting” You Didn’t Know You Needed: Just when you thought 3D printing was cool, scientists are already pushing into 4D bioprinting! This isn’t just about printing a static object; it’s about creating structures that can change shape or function over time in response to external stimuli like temperature, light, or pH. Imagine a bioprinted heart valve that can subtly adjust its shape as you grow, or a tissue scaffold that slowly dissolves and gets replaced by your own natural tissue, all while adapting to your body’s needs. It’s like printing with a built-in timer and smart responses, enabling dynamic integration with biological systems in ways we’ve never seen before. Truly mind-bending stuff that will lead to more robust and adaptive implants.
4. Ethical Debates Are as Important as Scientific Breakthroughs: As fascinating as the science is, let’s not forget the crucial conversations we need to have as a society. Questions around equitable access (who gets these amazing new organs?), the long-term implications of altering human biology, and the careful sourcing of biological materials are paramount. These aren’t just academic discussions; they’re vital for ensuring that bioprinting develops responsibly and benefits *all* of humanity, not just a select few. Engaging in these dialogues now helps shape policies that prevent future ethical dilemmas and ensures these revolutionary technologies are used for the greater good. It’s something I’m passionate about tracking, because technology should always serve humanity, not the other way around.
5. Following the Bioprinting Journey: If you’re as hooked on this topic as I am, there are some fantastic resources out there to keep you in the loop. Scientific journals like “Nature Biomedical Engineering” or “Science Translational Medicine” often feature groundbreaking studies. For more accessible news, tech and science blogs often have great summaries. Organizations like the Regenerative Medicine Advanced Therapy (RMAT) designation from the FDA, or research groups at universities like Harvard’s Wyss Institute and Wake Forest Institute for Regenerative Medicine, are constantly pushing the envelope. Subscribing to newsletters from these institutions or following key researchers on platforms like X (formerly Twitter) can give you a real insider’s view into the cutting edge of this incredible field. It’s like having a front-row seat to the future of medicine!
중요 사항 정리
Alright, so if there’s one thing to take away from our deep dive into bioprinting, it’s that we are genuinely in a golden age of innovation. We’ve seen how critical the development of advanced bioinks is—these aren’t just inert materials; they’re smart, living scaffolds that need to play nice with our cells and mimic the body’s natural environment. We’ve also tackled the vascularization puzzle, which is arguably the biggest game-changer for creating viable, human-sized tissues and organs. Solving the challenge of providing a continuous blood supply is absolutely essential for these bioprinted constructs to survive and thrive long-term. And let’s not forget the incredible leaps in precision and resolution, allowing scientists to craft structures with breathtaking detail, getting us closer to replicating the micro-architecture of native tissues. Then there’s the ongoing work on ensuring cell viability and functionality, making sure these printed cells not only live but also mature and integrate seamlessly into the body. Finally, scaling up for clinical reality and navigating the ethical and regulatory landscapes are the ultimate hurdles. It’s a complex, multi-faceted journey, but the sheer dedication and ingenuity of researchers worldwide mean that the dream of a future free from organ shortages and full of personalized medical breakthroughs is closer than ever before. It’s a truly inspiring time to be alive and witness such transformative science!
Frequently Asked Questions (FAQ) 📖
Q: What exactly is bioprinting, and how is it different from regular 3D printing?
A: You know how a regular 3D printer builds up an object layer by layer using plastic or metal? Well, bioprinting takes that same concept but uses “bioinks” – a fancy term for materials containing living cells and biomaterials – to construct functional tissues and organs.
It’s truly mind-blowing! Instead of just creating a static, inanimate object, bioprinting aims to build something that lives, breathes, and can integrate with our own bodies.
I often tell people it’s like building with tiny living LEGOs, carefully placed in specific patterns to mimic the intricate architecture of our own tissues.
That’s the real game-changer: creating structures that aren’t just solid, but biologically active and responsive.
Q: We hear about “challenges” a lot. What’s the real deal with the biggest technical hurdles bioprinting faces right now?
A: Oh, trust me, the journey isn’t without its bumps, even with all the incredible progress! From what I’ve seen, one of the toughest nuts to crack is vascularization.
Think about it: our natural tissues have an incredibly complex network of blood vessels to deliver nutrients and oxygen. When you print a tissue, especially a larger one, getting those tiny blood vessels to form and function properly within the printed structure is absolutely critical for the cells to survive and thrive.
Without it, the inner cells essentially starve. Then there’s the monumental task of scaling up these creations to a human level, ensuring the printed organs are not only viable but also perfectly functional and safe to integrate with a living person.
It’s a marathon, not a sprint, but the ingenuity being applied to these problems is genuinely inspiring!
Q: This sounds amazing, but realistically, when can we expect bioprinted organs to be available for transplant patients?
A: That’s the million-dollar question, isn’t it? While the progress is phenomenal, we’re not quite at the point where you’ll see bioprinted hearts on the shelf next year.
My take on it is that we’re likely to see smaller, simpler tissues and organs, like skin grafts or cartilage, reach clinical trials and even widespread use much sooner, possibly within the next decade.
For complex, fully functional organs like a kidney or a liver, which have incredibly intricate structures and multiple cell types, it’s going to take more time – perhaps another 15 to 20 years, or even more, before they become a routine transplant option.
There are so many steps involved, from perfecting the printing process and ensuring long-term viability to navigating rigorous regulatory approvals. But believe me, the direction is crystal clear, and the future of medicine is definitely heading towards a world where organ shortages could become a painful memory.